“Opposite charges attract and opposite charges repel” is an old basic principle of physics that you may have heard in school, but your teacher may have been wrong.
Researchers from the University of Oxford's Department of Chemistry found that similar charged particles immersed in solutions were able to attract each other from long distances, depending on the solvent used and the sign of the charge.
The study was published in the journal Nature nanotechnology.
The researchers believe their study will change the way scientists think about processes such as how drugs and chemicals remain stable or how certain diseases develop. They also discovered a way to measure the properties of the electrical charge generated by solvents, which was previously thought to be impossible.
“I am really very proud of my graduate students, as well as the undergraduate students, who have all worked together to move the needle on this fundamental discovery,” said Madhavi Krishnan, a professor at the University of Oxford, who led the study.
Zhang Kang.
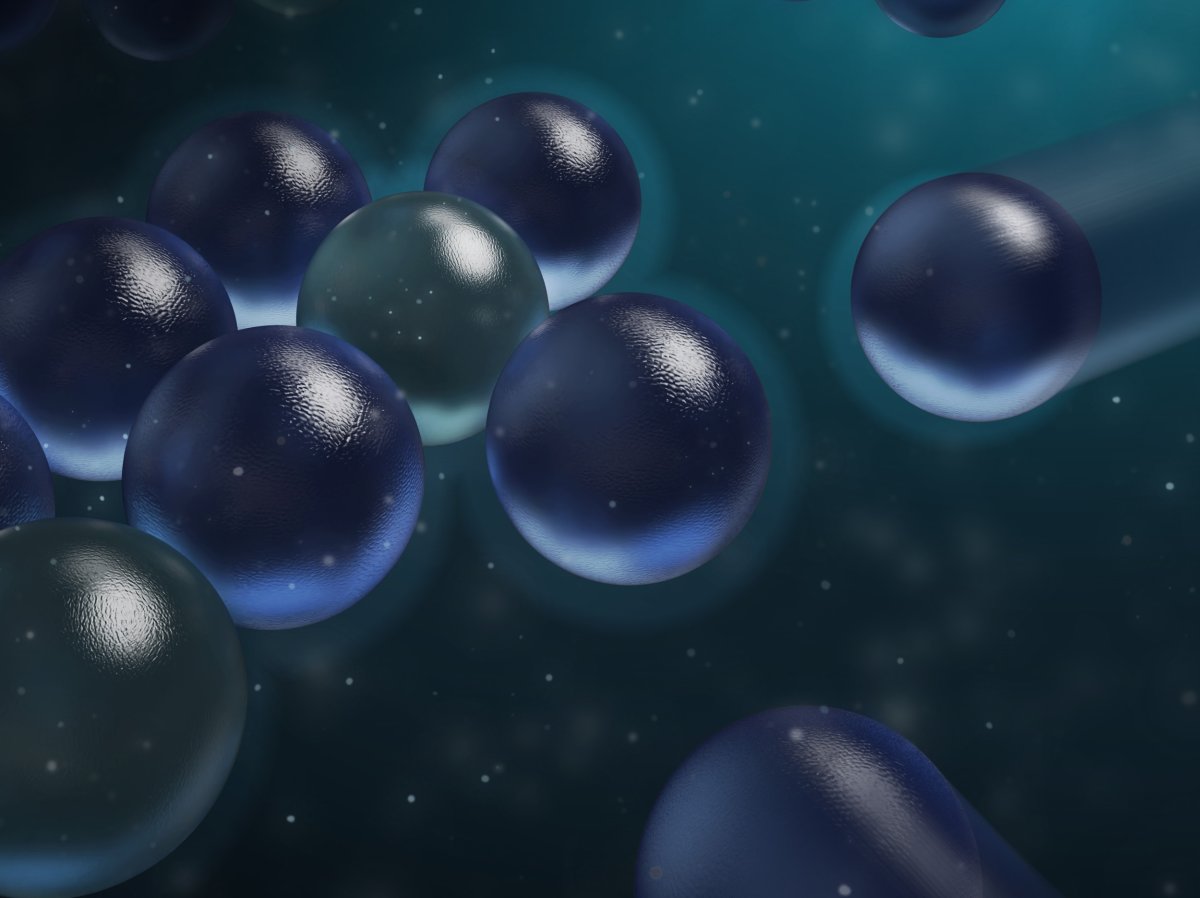
The researchers tracked tiny, negatively charged silica particles that were suspended in a solution, and discovered that these particles actually attract each other, forming hexagonally arranged clusters when they do so.
“I still find it fascinating to see these particles attracting each other, even after I've seen it a thousand times,” said Sidda Wang, first author of the study.
Although these negatively charged particles attract each other, positively charged particles do not.
Scientists believe that this phenomenon is caused by an attractive force found only in water that exceeds the usual electrostatic repulsion, allowing these clusters to form.
However, this attractive force had no effect on the positively charged particles in the water.
The scientists found that they were able to manipulate the formation of these clusters by changing the pH (acidity). However, regardless of pH, positively charged particles still cannot be attracted.
Throughout the study, the team also wondered whether the effect on these charged particles could be changed when the solvent was changed.
When they changed the solution to alcohol instead of water, they noticed that the positively charged silica particles formed these groups, while the negatively charged particles did not.
“Here we demonstrate experimentally that solvent plays a heretofore unsuspected but crucial role in interparticle interactions and, more importantly, that interactions in the liquid phase can break charge reversal symmetry,” the study authors wrote.
“We have shown that in aqueous solution, negatively charged particles can attract over a long distance while positively charged particles repel each other. In solvents that exhibit a pure molecular dipole inversion at the interface, such as alcohols, we find that the opposite can be true: particles may attract Positively charged particles repel each other.”
Updated 03/01/24, 06:14 AM ET: This article has been updated with additional information.
Uncommon knowledge
Newsweek is committed to challenging conventional wisdom and finding connections in the search for common ground.
Newsweek is committed to challenging conventional wisdom and finding connections in the search for common ground.

“Beer aficionado. Gamer. Alcohol fanatic. Evil food trailblazer. Avid bacon maven.”
